
Abiogenesis - Leading Clinical Research Organization.
Abiogenesis Clinpharm, a leading Southeast Asia CRO headquartered in Hyderabad, India, operates across the Philippines, Thailand, Vietnam, Bangladesh, Tanzania, and the Middle East. We support clinical trial innovations in drugs, nutraceuticals, and medical devices, advancing healthcare by delivering new treatment options through strategic clinical development.
Explore Our Main CRO Services
We support and guide pharmaceutical, biotechnology, medical device, herbal, and nutraceutical companies across all clinical trial phases. We understand the importance of selecting the right Clinical Research Organization (CRO) partner is critical to the success of your clinical research program. Abiogenesis has a team of clinical research professionals who are trusted to deliver services of the highest quality standards in compliance with all regulatory requirements. We comprise people with the right attitude, flexibility, reliability and commitment to excellence. Our commitment to project delivery is always based on evidence-based feasibilities.

Our Clinical Operations Team is experienced in managing clinical trials of varied size and complexity in different therapeutic areas

Our CDM team ensures accuracy & reliability of the clinical trial data in compliance with regulatory standards

We have a dedicated Quality Assurance Department to ensure that your projects will receive the highest level of quality in on time

Our Medical Affairs Department comprises of physicians, scientific writers and safety experts, And compliance with requirements
Our Biostatistics and Programming Team can manage complex studies by performing in-depth analysis of data in different therapeutic areas

Our Regulatory Affairs Department has competency in handling multiple regulatory activities from clinical trial applications
We Are The Trusted Clinical Research Experts
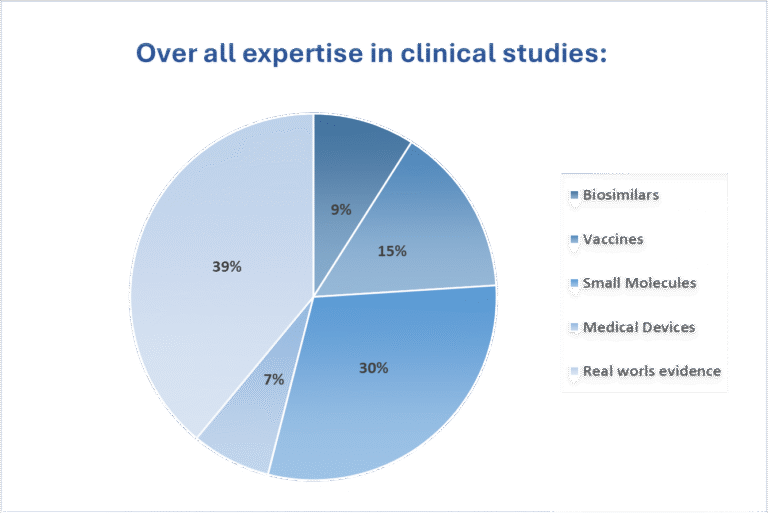
Our team has scientific experience and expertise in clinical trials of drugs, vaccines, biosimilars, medical devices, nutraceuticals, and herbal products. We provide comprehensive services, including selection of competent clinical sites and investigators, coordinate patient recruitment, safety surveillance, clinical monitoring, site audits, project management, data management and biostatistics. We are committed to carrying out clinical studies efficiently and effectively to meet regulatory guidelines and transform research findings into advanced treatments. We are well-versed with the traditional Phase trials as well as the recent advances in the industry like Real World Evidence studies , DeCentralized Trials and Ethnography research .
Biosimilar
Vaccine
Small molecule & FDC
110 +
Projects
35 +
Audits
30 +
Regulatory Studies
40 +
Clients
How Abiogenesis Will Help You
The successful execution and completion of a Clinical Trial is an enormous task involving clinical trial feasibility, financial support, retention and study design efforts that can take years to complete. It is established by a unified system that includes Medical Writing, Clinical Operations, Clinical Project management, Clinical Data Management, Statistical Analysis, Site Management, Regulatory Affairs, Pharmacovigilance, Quality Assurance and beyond.
HOW DOES AB HELP IN ACHIEVING YOUR CLINICAL STUDY GOALS
Abiogenesis Clinpharm is a Quality-driven Clinical Research Organization that can truly understand your needs and support you in accomplishing your objective. We strive to bring forth the best clinical trial services managed by our extensive knowledge and quality work experience in conducting your clinical trial.
What ethical and clinical practices does Abiogenesis Clinpharm follow in clinical research studies?
We are dedicated to tailoring the best ethical and clinical practices in the execution of clinical research studies, in addition to patient safety. Our aim is to provide customized clinical project management with accurate and reliable clinical data.
What services are included in Abiogenesis Clinpharm's clinical trial service portfolio?
Abiogenesis Clinpharm, CRO is open to all clients to provide a suite of clinical trial services
- Project Management
- Clinical Operations
- Regulatory Affairs
- Medical Affairs
- Clinical Data Management
- Quality Assurance
- Biostatistics
- Pharmacovigilanc
- FSP
How does Abiogenesis Clinpharm contribute to better patient clinical outcomes in the healthcare system?
We at Abiogenesis Clinpharm aim to advance clinical trials that enhance patient lives through holistic approaches, focus on patient-centric needs, utilize cutting-edge technology and most importantly, abide by stringent safety measures, data management practices, regulations and guidelines. With approaches like patient-centric trial designs, increased engagement strategies of patients, focus on ensuring safety and improving diversity with technology solutions in clinical trials, Abiogenesis can contribute significantly towards better health outcomes.
Do You Have Any Project?
Let's Talk About Solutions
CONTACT US
Industry Insights & Blogs

Abiogenesis Clinpharm: Success Stories & Milestones of a Leading Asian Clinical Research Organization (CRO)
Abiogenesis Clinpharm is a science-driven, quality-focused Clinical Research Organization (CRO) with over a decade of experience in delivering end-to-end clinical trial solutions across Asia and beyond. As a trusted partner in the clinical research ecosystem, we are committed to blending global standards with regional expertise, rooted in scientific integrity, patient safety, and operational excellence. Our active participation in prestigious events such as Bio Asia, Bio Korea, and ISCR reflect our commitment to staying at the forefront of scientific innovation and industry engagement.
Our Clinical Research Organization Journey: From One Desk to PAN-Asia (2014–2025)
- registered 2014 – Humble Beginnings
Every great story starts small. Our journey began with just one person and a vision. A visionary founder setting the stage for what would become a trusted CRO across Asia. - 2015 – First Milestones, First Impact
We made our mark by successfully executing our first Phase-III clinical trial, a dual achievement in both biosimilars and vaccines. A strong beginning that showcased our ability to handle complex studies with confidence. - 2016 – Powering Our Core
We brought everything in-house: clinical data management, medical writing, biostatistics, and regulatory affairs, consolidating our services to ensure seamless delivery under one roof. This year also marked our evolution into a full-fledged CRO, expanding beyond studies to strategic innovation. - 2017 – Global-Standard Validation
Flawless ANDA Study & Sites, audited by the USFDA and completed without a single 483. - 2018 – Breaking New Ground
Expanded into new domains by initiating Medical Device and Real-World Evidence studies, completing large-scale RWE projects involving over 10,000 subjects. - 2019 – Stepping into Asia’s Clinical Arena
Expanded our footprint to the Philippines, strengthening our identity as a truly Asia-focused CRO. - 2020 – Pandemic Response & Impact
Fast-tracked multiple vaccine trials — including critical COVID-19 studies — showcasing our strong commitment and ability to deliver reliable research even during tough times. - 2021 – Quality Meets Compliance
Achieved critical ISO certifications, including: ISO 9001:2015 - 2022 – Industry Recognition
Proudly listed among Outlook Magazine’s Top 10 Pharma Research Companies in India. A proud moment that proves our hard work and dedication. - 2023 – Digital Leap
Introduced our in-house e-CRF platform, transforming clinical data management with greater speed, intelligence, and efficiency — paving the way for next-gen digital trials.
ISO Certifications: | ISO 14155:2020 | ISO 27001:2022 |
- 2024 – Leading with Innovation
Awarded Best Emerging CRO of the Year in Vaccine Research at the Vaccine Leaders Conclave. A strong reflection of our rising leadership in vaccine research. - 2025 – CRO Registration Milestone
Abiogenesis Clinpharm successfully registered as a CRO via the SUGAM Portal, complying with CDSCO guidelines and reinforcing our commitment to regulatory excellence and clinical research innovation. registration via the SUGAM Portal, complying with CDSCO guidelines, reinforcing our commitment to regulatory excellence and clinical research innovation.
Meet the Visionaries Behind Abiogenesis Clinpharm’s Success
At Abiogenesis Clinpharm, our strength lies not only in world-class clinical trial services but in the visionary leadership that drives our success. With decades of combined expertise in clinical operations, medical affairs, technology, and executive strategy, our leadership team ensures quality, innovation, and excellence in every trial.
Pawan Bhusari – Chief Executive Officer
Pawan Bhusari brings 25+ years of leadership in clinical research, with expertise in end-to-end drug development. He has managed Phase I–IV trials and was instrumental in building robust clinical research infrastructures. His strategic mindset and deep industry knowledge have driven the success of efficient and outcome-focused trial systems.
Chikku Joseph – Chief Operating Officer
Chikku Joseph is a seasoned operations leader with over 20 years of experience in clinical research, holding a Master’s degree in Clinical Pharmacy. As COO, he is known for driving performance, leading global multi-country studies, and surpassing goals through visionary strategy, strong leadership, and operational excellence.
Dr. Antaryami Maharana – General Manager, Medical Affairs & PV
Dr. Antaryami Maharana is a highly experienced MD physician with 18+ years in clinical research, specializing in Phase I–IV trials. He has extensive expertise in setting up medical monitoring, medical writing, regulatory submissions, and pharmacovigilance systems, including case processing and the preparation of aggregate safety reports.
Ram Amancha: Chief Technology Officer
Ram Amancha is a seasoned IT professional with over 20 years of experience, having worked with prominent companies such as Dell, Walmart, and Cognizant. He brings deep expertise in high-tech, healthcare, and pharma sectors. At Abiogenesis, he drives innovation and growth in a rapidly evolving tech landscape.
Sahitya Pramod: Head Clinical Operations
A seasoned clinical research professional with 16+ years of experience in clinical operations, project management, and stakeholder engagement. Holds a Master’s in Clinical Research and a PG Diploma in Pharmaceutical Management. An early adopter of Adaptive Monitoring in a top 10 pharma company, with global experience at Novartis, Syneos Health, and Medidata.
Dr. Shalini Suraj: Assistant General Manager – Quality Assurance
Experienced QA professional with 16+ years in clinical research and 12+ years as an Auditor. A dentist by profession, skilled in GCP/GxP auditing across IND, ANDA, Phase 1–4, BA/BE, and medical device studies. Expert in clinical quality, vendor audits, and setting up QA departments with robust QMS and LMS systems.
Teja Duggineni: Sr. Manager – Clinical Data Management
13+ years of experience in Data Management across Phase I–IV clinical trials. Expert in setting up Data Management departments and systems for clinical research. Strong in project management, handling multiple projects with timely delivery.
Manohar Koppala: Head - RWE & RWD
Clinical Research Professional with 11+ years of experience in drug development, clinical trials, and RWE study management. Expert in Phase I–IV trials with 8 years in RWD/RWE. Proven track record in building and managing RWE & RWD departments and implementing robust, streamlined systems.
Key Achievements:
- Specialized CRO for Biosimilar Trials in India
Successfully executed numerous biosimilar clinical trials for molecules such as Denosumab, Rituximab, Adalimumab, Darbepoetin, Tocilizumab, and Trastuzumab. - Pioneers in Narcotic Drug Trials
Conducted a clinical trial involving Remifentanil, a narcotic analgesic, a rare and highly regulated category, showcasing the CRO’s capability in handling high-risk studies with precision. - Early Adopters of Real-World Evidence (RWE) Studies
Among the first Indian CROs to initiate Real World Studies since 2018, with nearly 35 RWE projects successfully completed. - USFDA-Audited ANDA Clinical Trial
Executed a landmark clinical ANDA study involving a small molecule, which was audited by the USFDA — one of the first-of-its-kind studies in India. - Comprehensive Vaccine Trial Expertise
Demonstrated robust capabilities in conducting vaccine trials for Dengue, Varicella, Liquid Hexavalent, Japanese Encephalitis, Measles-Rubella, and COVID-19, supporting submissions to DCGI, WHO, and other international regulatory bodies.
Operational Model Across PAN-ASIA
- Headquarters India – Central hub for strategic oversight, project management, and multi-country coordination.
- Sri Lanka, Bangladesh & Nepal – All operations are directly managed from India
- Tanzania – Clinical operations executed directly from a local base in Tanzania
- Philippines – On-ground clinical operations handled from a local base
- Vietnam & Thailand – Managed regionally from the Philippines base.
Full Suite of In-house Clinical Trial Services
We offer a comprehensive range of fully in-house clinical research services, ensuring seamless coordination, quality, and efficiency across all trial phases.
Our capabilities include:
- Clinical Operations
- Data Management
- Regulatory Affairs
- Medical & Allied Services
- Medical Devices
- Pharmacovigilance (PV)
- Real-World Evidence (RWE)
- Biostatistics & SAS Programming
- Quality Assurance & Audit
- Functional Service Provider (FSP) — PK/PD
Broad Therapeutic Area Expertise
We’ve successfully conducted studies across multiple therapeutic areas. Include:
- Neurology & Psychiatry
- Dermatology
- Infectious Diseases
- Cardiology
- Endocrinology
- Oncology
- Gynaecology
- Gastroenterology
- Nephrology & Urology
- Rheumatology
- Orthopaedics
- Ophthalmology
- Surgical Interventions
- Respiratory Disorders
- Parenteral Nutrition Support
Comprehensive Clinical Trial Experience
With a proven track record in diverse clinical research, we bring unmatched expertise across all phases and therapeutic domains.
Our extensive experience includes:
- 110+clinical trials successfully executed across India, the EU, and the USA
- 30+ studies approved by regulatory authorities
- 10+ Regulatory audits completed with excellence
- 40+ clients served globally, including top sponsors from India, Europe, and the United States
- 35+ Real-World Evidence (RWE) studies
Clinical Development Phase Experience
Experienced in managing clinical trials across all phases:Phase I: 6 studies | Phase II: 2 studies | Phase II/III: 3 studies | Phase III: 40studies | Phase IV: 15 studies | PMS: 7 studies | Medical Device: 3 studies
Comprehensive Clinical Expertise Across Modalities
We have expertise in biosimilar development, small molecule innovation, vaccine trials, medical device evaluations, and Real-World Evidence studies. Our work spans from early-phase designs to real-world data integration, ensuring regulatory-aligned, patient-focused, and technology-enabled trial execution across diverse therapeutic areas.
Certified Excellence at Abiogenesis Clinpharm
- ISO 14155:2020
Certified for Good Clinical Practice in medical device studies, ensuring ethical and scientific quality in human subject research. - ISO 9001:2015
Recognized for a robust Quality Management System, ensuring consistent, high-quality execution of clinical research services. - ISO/IEC 27001:2022
Certified for Information Security Management, assuring data privacy, cybersecurity, and regulatory compliance.
Recognitions & Industry Accolades
- Best Emerging CRO of the Year – 2024
Honored at the India Vaccine Leaders Conclave for our innovation, growth path, and impact in clinical research services. - Top 10 Pharma Research Companies – 2022
Recognized for our dedicated clinical expertise, global regulatory alignment, and ability to manage large-scale, multi-country trials with precision. - CEO Story Award – Company of the Year 2020
Appreciated our strategic leadership, client-focused approach, and adaptability during the COVID-19 pandemic. - Featured in Fortune Magazine
Spotlighted for conducting high-quality clinical trials, and our commitment to advancing new-age therapeutics with operational excellence.
Conclusion: Partner with Abiogenesis Clinpharm
With over a decade of proven excellence, Abiogenesis Clinpharm stands as a trusted CRO across biosimilars, vaccines, medical devices, and RWE. Our strength lies in quality, innovation, and PAN-Asia operational reach.
Connect with us:
Email [email protected] for collaborations or study discussions.
Let’s drive the future of clinical research—together.
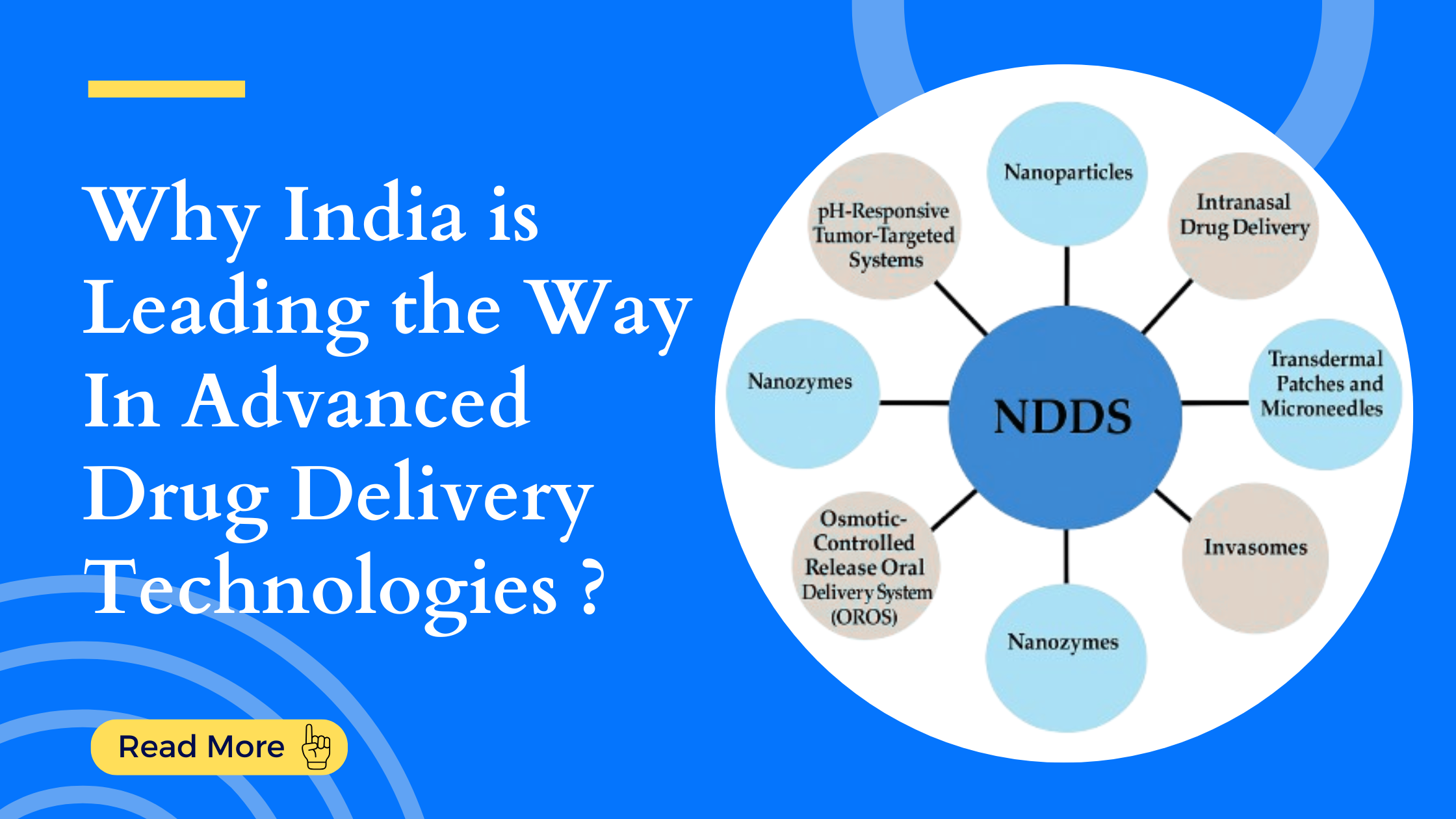
Targeted Therapies: Why India is Leading the Way in Novel Drug Delivery Technologies
India is rapidly emerging as a global leader in novel drug delivery technologies due to its large, diverse patient population, skilled researchers, cost-effective infrastructure, and a robust healthcare ecosystem. These factors are propelling India to the forefront of targeted drug delivery systems and clinical research innovations.
What Are Novel Drug Delivery Technologies (NDDS)?
Novel Drug Delivery Systems (NDDS) are advanced methods developed to deliver drugs in a more efficient, targeted, and controlled way. The main goals of NDDS include:
Enhanced drug targeting to specific tissues or organs
Controlled and sustained drug release
Reduced side effects and toxicity
Improved patient compliance and outcomes
Increased drug bioavailability
NDDS utilizes advanced tools like nanoparticles, liposomes, microneedles, and bioprinting to deliver drugs precisely and safely, transforming the landscape of pharmaceutical development and personalized medicine.
Types of Novel Drug Delivery Technologies
1. Nanoparticles
Tiny particles (1–100 nanometers) used to transport drugs directly to disease sites.
Made from lipids, polymers, or metals.
Offer high absorption and targeted drug release.
2. Nanozymes
Synthetic enzyme-like particles made from nanoparticles.
Useful in cancer therapy and detoxification by mimicking natural enzymes.
Allow for precise, low-toxicity treatments.
3. Intranasal Drug Delivery
Administered via nasal sprays, drops, or powders.
Enables rapid absorption through nasal blood vessels.
Bypasses the digestive system for faster action.
4. Transdermal Patches and Microneedles
Deliver drugs through the skin, reducing the need for injections.
Non-invasive, painless, and provide sustained release.
Ideal for chronic pain, hormone therapy, and vaccines.
5. Invasomes
Made from phospholipids, terpenes, and ethanol.
Designed to improve drug absorption through the skin.
Effective for drugs that don’t penetrate easily.
6. Ultrasound-Triggered Hydrogels
Hydrogels release drugs when exposed to ultrasound waves.
Useful for site-specific delivery, such as targeting tumors.
Reduces side effects by focusing the drug where it's needed.
7. Magnetic Electrospun Fibers
Contain drugs and magnetic nanoparticles within fibers.
Drug release is remotely triggered using magnetic fields.
Beneficial for oncology, wound care, and pain management.
8. Bioprinting
Uses 3D printing with living cells and bio-inks.
Creates tissue models for personalized drug testing.
Reduces the need for animal testing and improves drug trial accuracy.
9. pH-Responsive Tumor-Targeted Systems
Activate only in acidic environments, like tumor sites.
Ensure minimal impact on healthy tissues.
Improve cancer therapy outcomes.
10. Osmotic-Controlled Release Oral Systems (OROS)
Tablet systems that use water-driven pressure for steady drug release.
Maintain consistent drug levels in the bloodstream.
Improve patient adherence and therapeutic effects.
Traditional Drug Delivery Methods in India: A Look Back
Historically, India’s clinical trials used conventional drug delivery forms such as:
Oral Tablets and Capsules – Most common method for systemic effects.
Injections – Intravenous or intramuscular for quick results.
Topical Creams and Gels – For localized skin treatments.
Challenges with Traditional Methods:
Systemic side effects due to poor targeting
Low bioavailability and fast drug degradation
Frequent dosing required
First-pass metabolism reducing drug potency
How NDDS Is Transforming Clinical Trials in India
Novel Drug Delivery Systems are revolutionizing clinical trials by improving how drugs interact with the human body during testing.
✅ Benefits of NDDS in Clinical Trials:
Improved Targeting: Focuses drug action on specific sites (e.g., tumors).
Reduced Side Effects: Lowers systemic exposure and enhances safety.
Better Compliance: Controlled release reduces the number of doses needed.
Accurate Results: Uniform drug delivery provides consistent trial outcomes.
Enables Complex Therapies: Supports delivery of biologics, mRNA, or unstable molecules.
Why India Is Leading the Way in Advanced Drug Delivery
India’s leadership in novel drug delivery technologies is driven by:
Skilled pharmaceutical and biotech talent
Strong CROs like Abiogenesis Clinpharm
Lower trial costs without compromising quality
Access to large patient pools for rapid recruitment
Supportive regulatory frameworks and global partnerships
Abiogenesis Clinpharm: Driving Innovation in Drug Delivery Trials
At Abiogenesis Clinpharm, we are committed to advancing global healthcare by supporting clinical trials that involve targeted therapies and novel drug delivery systems. Our dedicated team works with cutting-edge technologies like nanoparticles, microneedles, and 3D bioprinting to bring more precise, safer, and effective treatments to patients worldwide.
We support sponsors across various therapeutic areas, ensuring regulatory compliance, robust data, and ethical research practices at every phase.
Conclusion
Novel Drug Delivery Technologies are not only improving how medicines are delivered but also how clinical trials are conducted—especially in a forward-thinking hub like India. These innovations are making treatments smarter, safer, and more personalized. As a trusted clinical research organization, Abiogenesis Clinpharm remains at the forefront of this transformation—empowering new possibilities in global drug development.
Let’s work together to redefine what’s possible in clinical research.


My Experience at Bio Korea 2025: Insights on Innovation, Technology & Global Collaborations
By Pawan Bhusari, CEO | Published June 2025
Introduction
From May 7th to 9th, I had the privilege of attending Bio Korea 2025, one of Asia’s most influential biotech and healthcare conferences, held at COEX in Seoul, South Korea. As a business development professional, this event was truly a revelation. It brought together cutting-edge global innovation, smart, tech-driven healthcare solutions, and robust business partnering opportunities, all within an exceptionally advanced and welcoming environment.
The exhibition floor buzzed with transformative ideas—from AI-powered diagnostics and decentralized clinical trials to next-generation biologics and digital therapeutics. Every booth and discussion reflected the global push toward precision medicine and patient-centric innovation.
What stood out to me was not just the scale of the conference but the quality of conversations and the collaborative mindset among participants. I had the opportunity to connect with clinical research organizations, biotech innovators, pharmaceutical companies, and government agencies—all looking to shape the future of healthcare together.
South Korea left a lasting impression. Beyond its biotech leadership, the country's world-class infrastructure, highly digitalized healthcare ecosystem, and forward-thinking regulatory landscape exemplify what it means to be future-ready in life sciences. It’s no surprise that Korea is becoming a global hub for clinical research and innovation.
Attending Bio Korea 2025 reaffirmed my belief in the power of global collaboration to drive meaningful change in healthcare. I return inspired, informed, and more committed than ever to advancing innovation through strategic partnerships.
Technology Trends That Stood Out at Bio Korea 2025
AI & Big Data in Drug Discovery
Cell & Gene Therapies
Smart Medical Devices
Digital Health Ecosystems
Green Biomanufacturing
Highlights from the Conference – Day-Wise Summary


Day 1: Exploring the Future of Medicine
AI-Based New Drug Development
Artificial intelligence is revolutionizing drug discovery. Korean and global companies showcased AI tools that predict compound behaviour, optimize clinical trial designs, and shorten R&D timelines.
Brain-Computer Interface (BCI)
We explored how neural signals are being used to control external devices. The possibilities for neuro-rehabilitation and assistive technologies are inspiring.
Regenerative Medicine
Advances in stem cell therapy, gene editing, and tissue engineering are transitioning from lab to life. Korea’s high-quality research in this domain is world-class.
Day 2: Global Collaboration & Scientific Breakthroughs
Open Innovation in Pharma
Sessions emphasized the value of collaboration between pharma, biotech, and academia to fast-track breakthroughs.
Global Bio Governance
A deep dive into how ethics, regulation, and international standards are evolving. Korea is aligning with global norms to enhance biotech governance.
Regenerative Medicine
Startups and government-backed initiatives are positioning Korea as a global hub in this domain.
New Drug Modalities
Topics included RNA-based drugs, antibody-drug conjugates, and personalized medicine – all pushing the boundaries of modern treatment.
Sustainable Global Biopharmaceutical Approvals
Regulatory science and sustainable strategies were discussed to streamline global approval processes.
Space Biotechnology
Biomedical research in microgravity and future bio-manufacturing in space were visionary highlights.
Day 3: Aging, Trials & Converging Tech
Anti-aging and Rejuvenation
Innovations in diagnostics, biomarkers, and immunotherapy showcased how aging can be slowed for healthier longevity.
Clinical Trials
Korea’s infrastructure and decentralized clinical trials (DCTs) were impressive. AI-based monitoring tools also stood out.
Preclinical – Alternative Toxicology
Focus on organ-on-chip models and AI-driven toxicology as ethical, efficient alternatives to animal testing.
Reverse-Aging Technologies
Sessions highlighted senolytics, longevity-based gene editing, and future-forward regenerative approaches.
Bio-Digital Convergence Technology
The convergence of AI, digital twins, and cloud-based diagnostics with biotech was an inspiring end to the conference.
Business Partnering: A Global Collaboration Hub
One of the most valuable aspects was the Bio Korea 2025 Business Partnering Event, a seamless B2B platform for 1:1 meetings:
Connected with CROs, biotech leaders, regulatory experts, and investors from the U.S., EU, and APAC.
Explored AI-driven clinical trial platforms, regulatory consulting, and out-licensing opportunities.
Each meeting provided real-world insights and strategic value.
Why South Korea Is a Model for Biotech Development
Digitally Advanced Nation
- Fully digitized systems in transport, healthcare, and business.
- Real-time data and AI-driven decision-making.
Regulatory Vision
- MFDS is globally aligned and innovation-friendly.
- Strong infrastructure for clinical trials and global collaboration.
Government R&D Support
- Grants, tax incentives, biotech zones, and innovation parks.
- Target: Make Korea Asia’s top biotech hub by 2030.
Safe, Smart, Systematic
- 5G-enabled hospitals, AI triage, and clean, organized cities ideal for international business.
Conclusion: A Game-Changer for Clinical Research & Innovation
Bio Korea 2025 was more than a conference – it was a gateway to the future of healthcare. For Abiogenesis Clinpharm, it opens new avenues for regulatory alignment, innovation, and strategic international collaboration.
I return from Seoul with:
- Actionable leads
- Strategic partnerships
- A fresh perspective on integrating global biotech trends into our vision

