We at Abiogenesis are committed to drive your clinical trial program in healthcare through our specialized expertise in biosimilar clinical development. Our team is designed to serve as a focal point for pharmaceutical companies looking to navigate the complex and evolving landscape of biosimilars clinical trials. With years of experience in the field of biosimilars, our team brings a wealth of knowledge and a successful track record of guiding biosimilar projects from study synopsis to approval. We understand the stringent regulatory requirements governing biosimilar development and our team work closely with entire team to ensure your trials meet all compliance standards. We offer end-to-end clinical trial management, encompassing site selection, patient recruitment, data management, and regulatory submissions for your entire program.
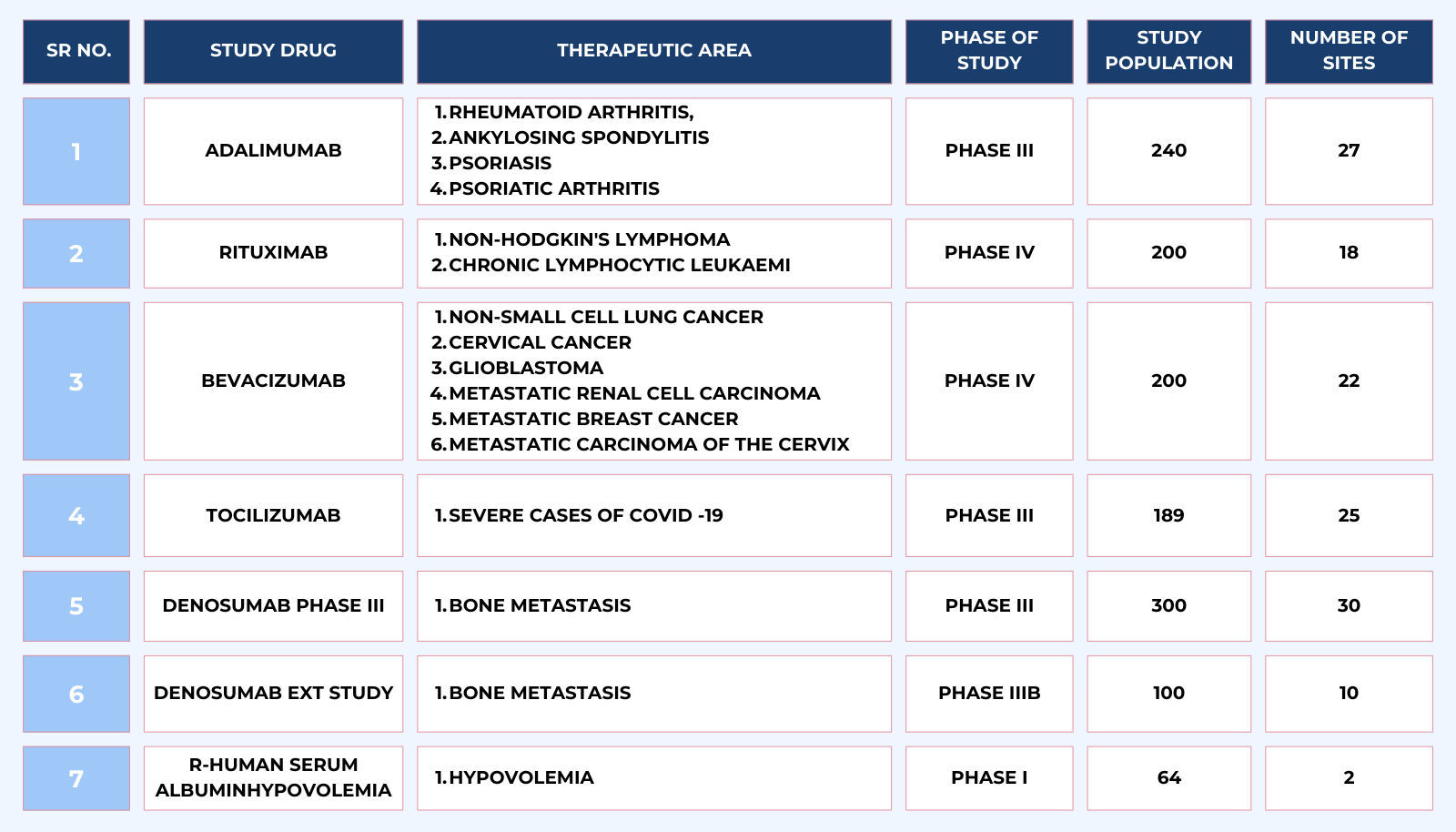
There are many studies we have worked on many biosimilars and few of them are list below. Let us know how we can contribute in your biosimilar clinical development program.