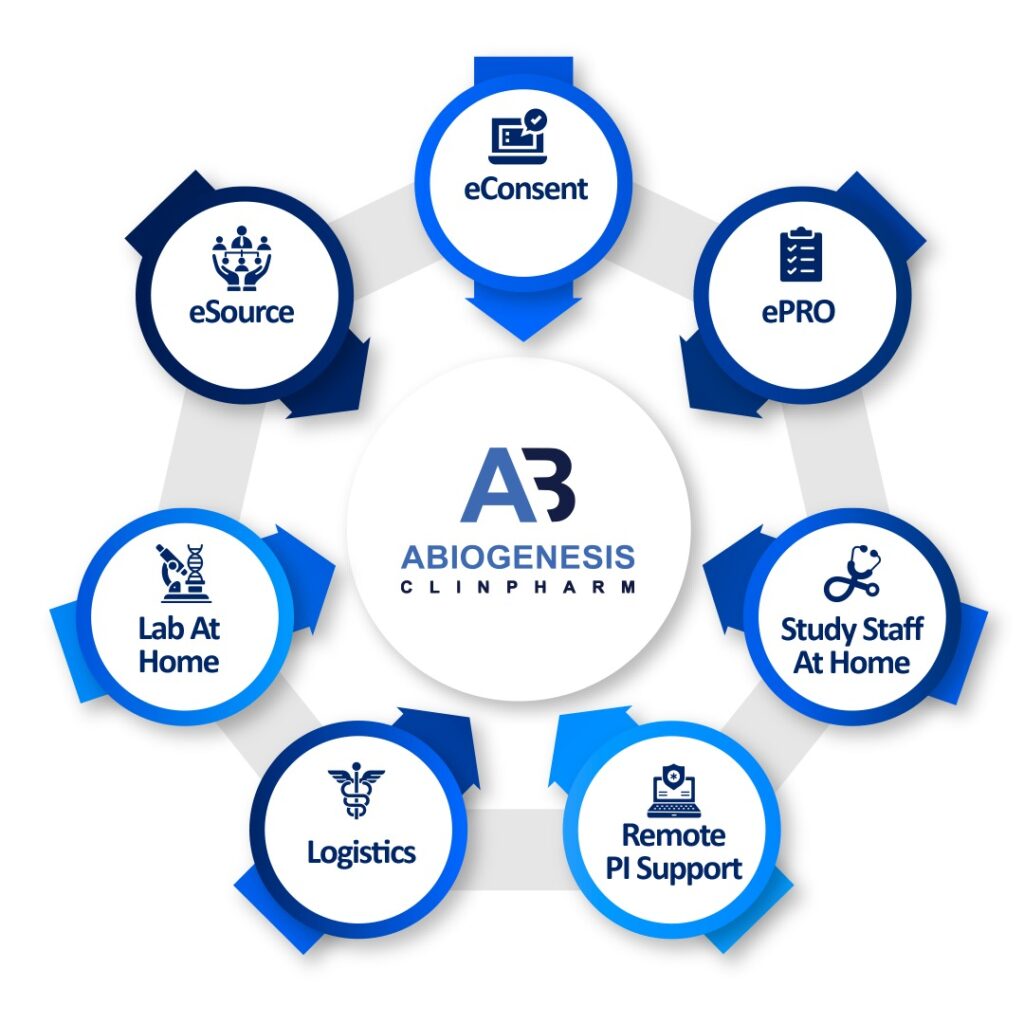
Decentralized clinical trials (DCTs), also known as virtual or remote clinical trials, represent a modern approach to conducting clinical research. These trials aim to improve the efficiency, inclusivity, and patient-centric nature of clinical trials by leveraging technology and reducing the need for patients to visit physical trial sites. Here are some key aspects of decentralized clinical trials where Abiogenesis can help you execute your DCT.
Remote Participation
Technology Utilization
Data Collection
Patient-Centric Approach
Inclusivity
Enhanced Efficiency
Remote Participation
Technology Utilization
Data Collection

Patient-Centric Approach
Inclusivity
Enhanced Efficiency
We as an Abiogenesis are working with many decentralized Clinical Trials where our services are integrated with advanced technologies like CTMS, e-CRF, and e-PRO along with other technological advancements where we can effectively do the DCT along with applicable regulation. If you are looking for decentralized clinical trial services, then please reach out to us for further information.


