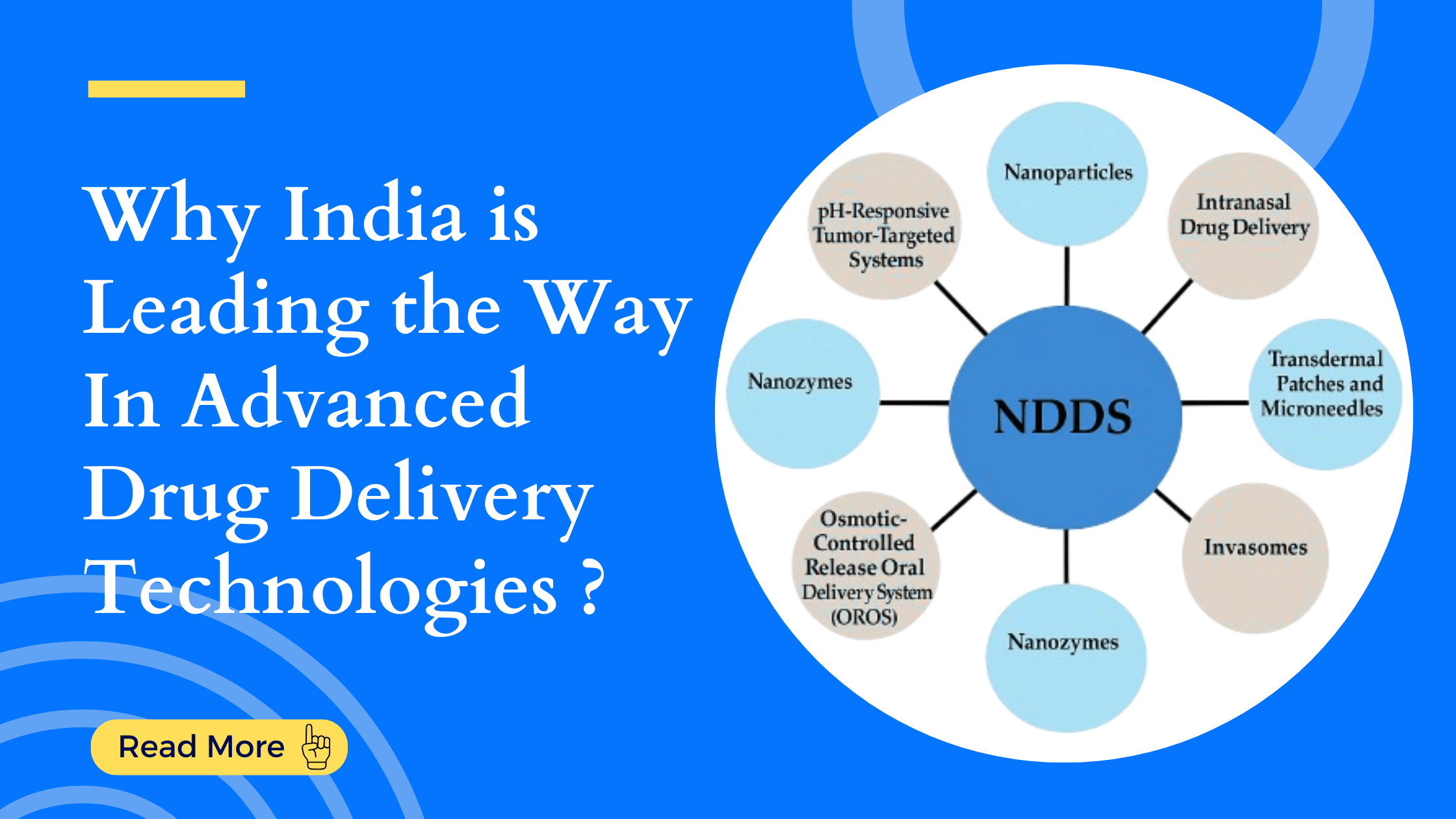
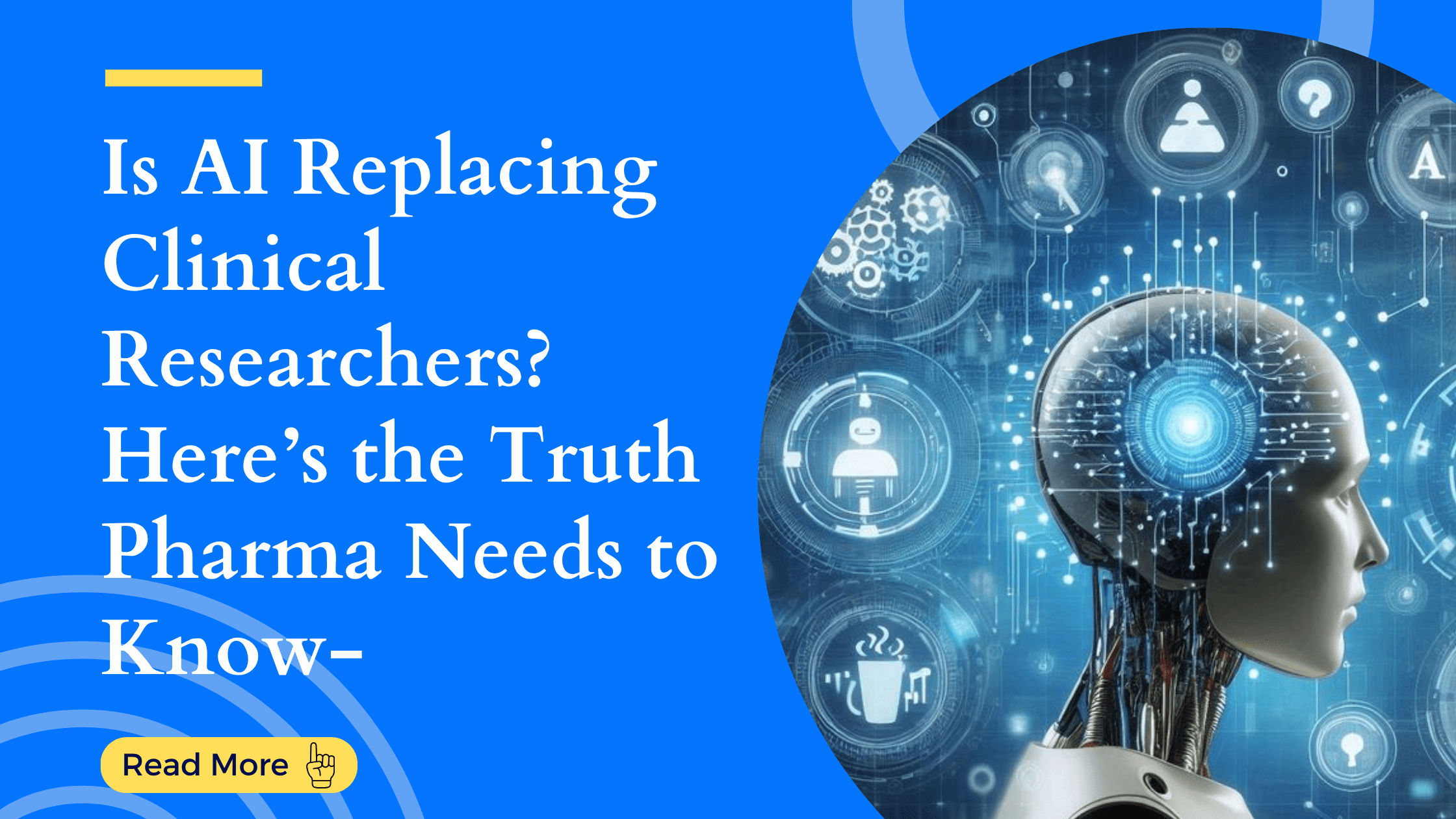
Strategic CRO Selection: Transforming Trials into Triumphs Running a clinical trial is an exci...
16
Feb

Date February 16, 2026
Tags clinical research companies in hyderabad, Clinical Research Companies in India, Clinical Research Organization in Hyderabad, Clinical Research Organization in India, Clinical Research Organizations, Clinical Trial Management, Clinical Trial Services, CRO Selection Guide, Top 10 Cros In India, Top Clinical Research Organizations in India Categories Biostastics, Clinical Operations, Clinical Research Organization, Data Management, Medical Affairs, Quality Assurance, Regulatory Affairs