Abiogenesis Clinpharm: Success Stories & Milestones of a Leading Asian Clinical Research Organization (CRO)
Abiogenesis Clinpharm is a science-driven, quality-focused Clinical Research Organization (CRO) with over a decade of experience in delivering end-to-end clinical trial solutions across Asia and beyond. As a trusted partner in the clinical research ecosystem, we are committed to blending global standards with regional expertise, rooted in scientific integrity, patient safety, and operational excellence. Our active participation in prestigious events such as Bio Asia, Bio Korea, and ISCR reflect our commitment to staying at the forefront of scientific innovation and industry engagement.
Our Clinical Research Organization Journey: From One Desk to PAN-Asia (2014–2025)
Registered 2014 – Humble Beginnings
Every great story starts small. Our journey began with just one person and a vision. A visionary founder setting the stage for what would become a trusted CRO across Asia.
2015 – First Milestones, First Impact
We made our mark by successfully executing our first Phase-III clinical trial, a dual achievement in both biosimilars and vaccines. A strong beginning that showcased our ability to handle complex studies with confidence.
2016 – Powering Our Core
We brought everything in-house: clinical data management, medical writing, biostatistics, and regulatory affairs, consolidating our services to ensure seamless delivery under one roof. This year also marked our evolution into a full-fledged CRO, expanding beyond studies to strategic innovation.
2017 – Global-Standard Validation
Flawless ANDA Study & Sites, audited by the USFDA and completed without a single 483.
2018 – Breaking New Ground
Expanded into new domains by initiating Medical Device and Real-World Evidence studies, completing large-scale RWE projects involving over 10,000 subjects.
2019 – Stepping into Asia’s Clinical Arena
Expanded our footprint to the Philippines, strengthening our identity as a truly Asia-focused CRO.
2020 – Pandemic Response & Impact
Fast-tracked multiple vaccine trials — including critical COVID-19 studies — showcasing our strong commitment and ability to deliver reliable research even during tough times.
2021 – Quality Meets Compliance
Achieved critical ISO certifications, including: ISO 9001:2015
2022 – Industry Recognition
Proudly listed among Outlook Magazine’s Top 10 Pharma Research Companies in India. A proud moment that proves our hard work and dedication.
2023 – Digital Leap
Introduced our in-house e-CRF platform, transforming clinical data management with greater speed, intelligence, and efficiency — paving the way for next-gen digital trials.
ISO Certifications: | ISO 14155:2020 | ISO 27001:2022 |
2024 – Leading with Innovation
Awarded Best Emerging CRO of the Year in Vaccine Research at the Vaccine Leaders Conclave. A strong reflection of our rising leadership in vaccine research.2025 – CRO Registration Milestone
Abiogenesis Clinpharm successfully registered as a CRO via the SUGAM Portal, complying with CDSCO guidelines and reinforcing our commitment to regulatory excellence and clinical research innovation. registration via the SUGAM Portal, complying with CDSCO guidelines, reinforcing our commitment to regulatory excellence and clinical research innovation.
Meet the Visionaries Behind Abiogenesis Clinpharm’s Success
At Abiogenesis Clinpharm, our strength lies not only in world-class clinical trial services but in the visionary leadership that drives our success. With decades of combined expertise in clinical operations, medical affairs, technology, and executive strategy, our leadership team ensures quality, innovation, and excellence in every trial.
Pawan Bhusari – Chief Executive Officer
Pawan Bhusari brings 25+ years of leadership in clinical research, with expertise in end-to-end drug development. He has managed Phase I–IV trials and was instrumental in building robust clinical research infrastructures. His strategic mindset and deep industry knowledge have driven the success of efficient and outcome-focused trial systems.
Chikku Joseph – Chief Operating Officer
Chikku Joseph is a seasoned operations leader with over 20 years of experience in clinical research, holding a Master’s degree in Clinical Pharmacy. As COO, he is known for driving performance, leading global multi-country studies, and surpassing goals through visionary strategy, strong leadership, and operational excellence.
Dr. Antaryami Maharana – General Manager, Medical Affairs & PV
Dr. Antaryami Maharana is a highly experienced MD physician with 18+ years in clinical research, specializing in Phase I–IV trials. He has extensive expertise in setting up medical monitoring, medical writing, regulatory submissions, and pharmacovigilance systems, including case processing and the preparation of aggregate safety reports.
Ram Amancha: Chief Technology Officer
Ram Amancha is a seasoned IT professional with over 20 years of experience, having worked with prominent companies such as Dell, Walmart, and Cognizant. He brings deep expertise in high-tech, healthcare, and pharma sectors. At Abiogenesis, he drives innovation and growth in a rapidly evolving tech landscape.
Sahitya Pramod: Head Clinical Operations
A seasoned clinical research professional with 16+ years of experience in clinical operations, project management, and stakeholder engagement. Holds a Master’s in Clinical Research and a PG Diploma in Pharmaceutical Management. An early adopter of Adaptive Monitoring in a top 10 pharma company, with global experience at Novartis, Syneos Health, and Medidata.
Dr. Shalini Suraj: Assistant General Manager – Quality Assurance
Experienced QA professional with 16+ years in clinical research and 12+ years as an Auditor. A dentist by profession, skilled in GCP/GxP auditing across IND, ANDA, Phase 1–4, BA/BE, and medical device studies. Expert in clinical quality, vendor audits, and setting up QA departments with robust QMS and LMS systems.
Teja Duggineni: Sr. Manager – Clinical Data Management
13+ years of experience in Data Management across Phase I–IV clinical trials. Expert in setting up Data Management departments and systems for clinical research. Strong in project management, handling multiple projects with timely delivery.
Manohar Koppala: Head - RWE & RWD
Clinical Research Professional with 11+ years of experience in drug development, clinical trials, and RWE study management. Expert in Phase I–IV trials with 8 years in RWD/RWE. Proven track record in building and managing RWE & RWD departments and implementing robust, streamlined systems.
Key Achievements:
Specialized CRO for Biosimilar Trials in India
Successfully executed numerous biosimilar clinical trials for molecules such as Denosumab, Rituximab, Adalimumab, Darbepoetin, Tocilizumab, and Trastuzumab.Pioneers in Narcotic Drug Trials
Conducted a clinical trial involving Remifentanil, a narcotic analgesic, a rare and highly regulated category, showcasing the CRO’s capability in handling high-risk studies with precision.Early Adopters of Real-World Evidence (RWE) Studies
Among the first Indian CROs to initiate Real World Studies since 2018, with nearly 35 RWE projects successfully completed.USFDA-Audited ANDA Clinical Trial
Executed a landmark clinical ANDA study involving a small molecule, which was audited by the USFDA — one of the first-of-its-kind studies in India.Comprehensive Vaccine Trial Expertise
Demonstrated robust capabilities in conducting vaccine trials for Dengue, Varicella, Liquid Hexavalent, Japanese Encephalitis, Measles-Rubella, and COVID-19, supporting submissions to DCGI, WHO, and other international regulatory bodies.
Operational Model Across PAN-ASIA
- Headquarters India – Central hub for strategic oversight, project management, and multi-country coordination.
- Sri Lanka, Bangladesh & Nepal – All operations are directly managed from India
- Tanzania – Clinical operations executed directly from a local base in Tanzania
- Philippines – On-ground clinical operations handled from a local base
- Vietnam & Thailand – Managed regionally from the Philippines base.
Full Suite of In-house Clinical Trial Services
We offer a comprehensive range of fully in-house clinical research services, ensuring seamless coordination, quality, and efficiency across all trial phases.
Our capabilities include:
- Clinical Operations
- Data Management
- Regulatory Affairs
- Medical & Allied Services
- Medical Devices
- Pharmacovigilance (PV)
- Real-World Evidence (RWE)
- Biostatistics & SAS Programming
- Quality Assurance & Audit
- Functional Service Provider (FSP) — PK/PD
Broad Therapeutic Area Expertise
We’ve successfully conducted studies across multiple therapeutic areas. Include:
- Neurology & Psychiatry
- Dermatology
- Infectious Diseases
- Cardiology
- Endocrinology
- Oncology
- Gynaecology
- Gastroenterology
- Nephrology & Urology
- Rheumatology
- Orthopaedics
- Ophthalmology
- Surgical Interventions
- Respiratory Disorders
- Parenteral Nutrition Support
Comprehensive Clinical Trial Experience
With a proven track record in diverse clinical research, we bring unmatched expertise across all phases and therapeutic domains.
Our extensive experience includes:
- 110+clinical trials successfully executed across India, the EU, and the USA
- 30+ studies approved by regulatory authorities
- 10+ Regulatory audits completed with excellence
- 40+ clients served globally, including top sponsors from India, Europe, and the United States
- 35+ Real-World Evidence (RWE) studies
Clinical Development Phase Experience
Experienced in managing clinical trials across all phases:Phase I: 6 studies | Phase II: 2 studies | Phase II/III: 3 studies | Phase III: 40studies | Phase IV: 15 studies | PMS: 7 studies | Medical Device: 3 studies
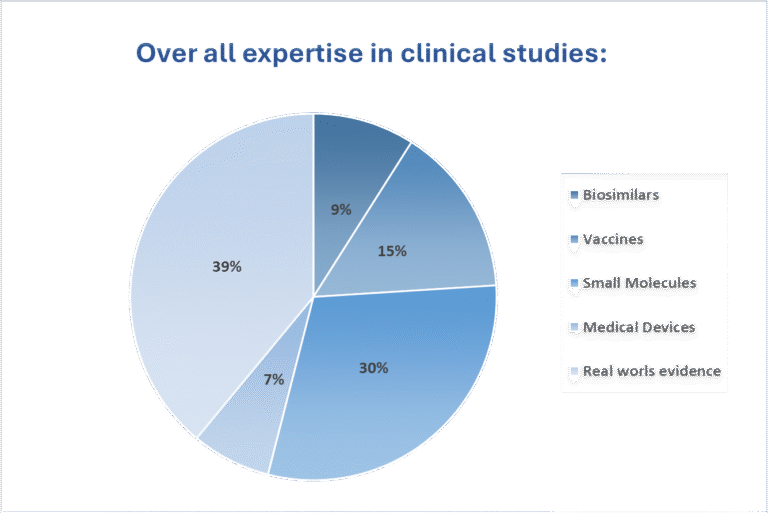
Comprehensive Clinical Expertise Across Modalities
We have expertise in biosimilar development, small molecule innovation, vaccine trials, medical device evaluations, and Real-World Evidence studies. Our work spans from early-phase designs to real-world data integration, ensuring regulatory-aligned, patient-focused, and technology-enabled trial execution across diverse therapeutic areas.
Certified Excellence at Abiogenesis Clinpharm
ISO 14155:2020
Certified for Good Clinical Practice in medical device studies, ensuring ethical and scientific quality in human subject research.ISO 9001:2015
Recognized for a robust Quality Management System, ensuring consistent, high-quality execution of clinical research services.ISO/IEC 27001:2022
Certified for Information Security Management, assuring data privacy, cybersecurity, and regulatory compliance.
Recognitions & Industry Accolades
Best Emerging CRO of the Year – 2024
Honored at the India Vaccine Leaders Conclave for our innovation, growth path, and impact in clinical research services.Top 10 Pharma Research Companies – 2022
Recognized for our dedicated clinical expertise, global regulatory alignment, and ability to manage large-scale, multi-country trials with precision.CEO Story Award – Company of the Year 2020
Appreciated our strategic leadership, client-focused approach, and adaptability during the COVID-19 pandemic.Featured in Fortune Magazine
Spotlighted for conducting high-quality clinical trials, and our commitment to advancing new-age therapeutics with operational excellence.
Conclusion: Partner with Abiogenesis Clinpharm
With over a decade of proven excellence, Abiogenesis Clinpharm stands as a trusted CRO across biosimilars, vaccines, medical devices, and RWE. Our strength lies in quality, innovation, and PAN-Asia operational reach.
Connect with us:
Email [email protected] for collaborations or study discussions.
Let’s drive the future of clinical research—together.



