Top 10 CROs in India: Why Abiogenesis Clinpharm Deserves Recognition When sponsors and pharmac...

Categories Clinical Research Organization, Biostastics
Data Visualization for Decision-Makers: Transforming Biostatistics Beyond Tables and Listings...

Categories Clinical Research Organization, Clinical Operations
Essential Documents in Clinical Trials: Ensuring Transparency and Compliance Clinical trials...
Essential Leadership: Why the Principal Investigator is Key in Clinical Trials Clinical trials...

Categories Clinical Research Organization, Clinical Operations
Understanding ICF in Clinical Trials: A Gateway to Ethical Participation In the world of clini...

Categories Clinical Research Organization, Clinical Operations
Revolutionizing Research: How Decentralized Clinical Trials Are Shaping the Future of Drug Developme...

Categories Clinical Research Organization
Abiogenesis Clinpharm: Success Stories & Milestones of a Leading Asian Clinical Research Organiz...
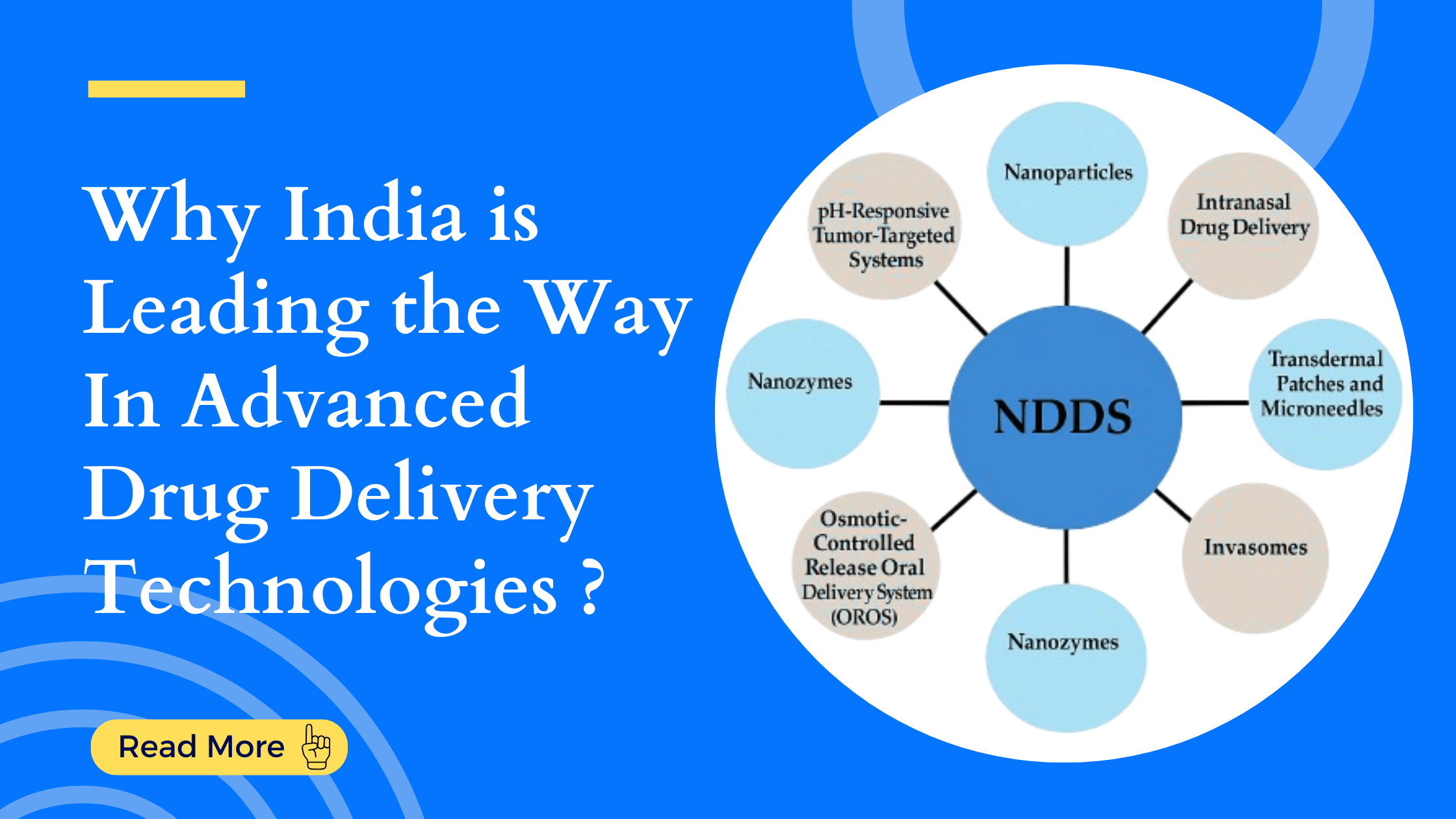
Targeted Therapies: Why India is Leading the Way in Novel Drug Delivery Technologies India is...

Insights from Bio Korea 2025: How South Korea is Shaping the Future of Global Healthcare & Biote...



