What is a CRO? | What is Clinical Trial Participation?
A CRO or Clinical Research Organization is a company that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services. CROs help conduct clinical trials, manage regulatory requirements, handle data analysis, and more.
They play a key role in bringing new drugs and medical devices to market faster and more efficiently. CROs work with sponsors (like pharma companies) to design, manage, and monitor clinical studies while ensuring compliance with global regulatory standards.
Key services offered by a CRO include:
Clinical trial management
Site selection and monitoring
Data management and biostatistics
Regulatory affairs
Medical writing
Pharmacovigilance
Why CROs are Important:
They reduce the burden on pharmaceutical companies by providing expert services and infrastructure needed to run clinical trials effectively.
What is Clinical Trial Participation?
Clinical Trial Participation refers to the involvement of volunteers (patients or healthy individuals) in research studies that evaluate new medical treatments, drugs, or devices. These trials are essential for determining whether a new treatment is safe and effective before it can be approved for public use.
Participants play a critical role in advancing medical research and helping develop new therapies that can improve or save lives.
Types of participants:
Healthy volunteers (to understand how a drug behaves in the body)
Patients with specific medical conditions (to evaluate treatment effects)
What participants do:
Receive investigational treatments
Attend study visits and undergo tests
Share their health data with researchers
Follow specific trial protocols
Why clinical trial participation matters:
It contributes to scientific discovery
Participants may gain access to new therapies before they’re publicly available
Helps improve future treatments for others
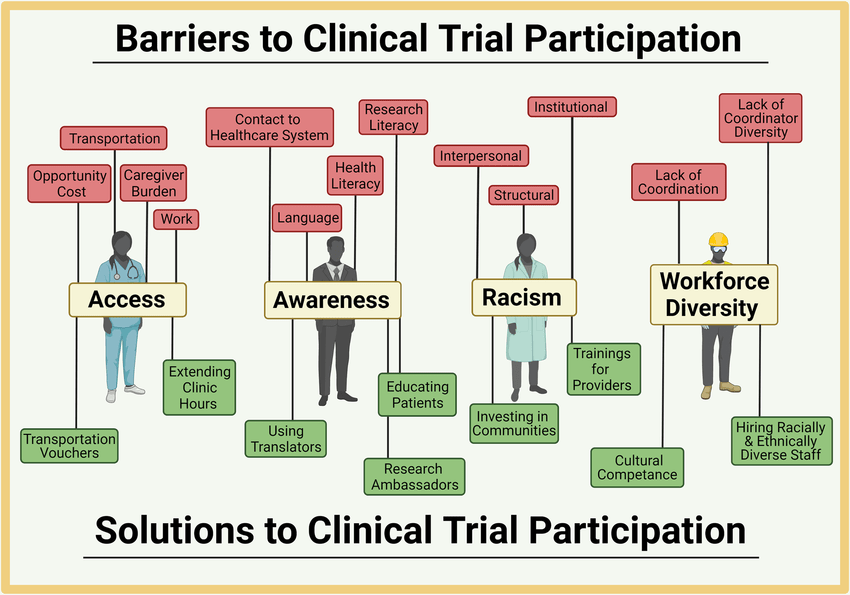
Navigating Barriers and Forging Solutions for Seamless Clinical Trial Participation
Clinical trials serve as the cornerstone of medical innovation, offering a pathway to test and validate new treatments before they reach the public. Yet, despite their critical role, challenges in clinical trial participation continue to hinder progress. From patient recruitment to regulatory bottlenecks, these issues impact trial timelines, increase costs, and compromise study diversity. At Abiogenesis Clinpharm, a trusted Clinical Research Organization in Hyderabad, we have adopted a patient-centric approach to dismantle these barriers and ensure streamlined trial operations.
Bridging the Awareness and Accessibility Gap
A primary barrier to participation is the lack of awareness among potential volunteers. Many individuals—especially from underserved communities—are unaware of the existence or relevance of clinical trials. Misinformation, historical mistrust, and the complexity of trial details further deepen the divide.
Solutions:
As a leading Clinical Research Organization in India, we emphasize culturally sensitive and community-focused outreach. Collaborating with patient advocacy groups and community health workers, Abiogenesis Clinpharm ensures that trial-related information is accessible, simplified, and available in local languages. We also utilize digital platforms and media outlets to reach a broader audience. Our centralized trial registries allow patients to find suitable trials effortlessly, helping close the information gap.
Minimizing Financial and Logistical Burdens
Travel requirements, time constraints, and out-of-pocket expenses often discourage participation. These burdens are particularly heavy for individuals in rural or economically disadvantaged regions.
Solutions:
As one of the top Clinical Research Organizations in India, Abiogenesis Clinpharm embraces decentralized trial models, telemedicine, and mobile health (mHealth) technologies. We also offer financial aid and logistical support, including transportation, accommodation, and flexible scheduling. These efforts make trial participation feasible and less stressful for patients across diverse demographics.
Simplifying Complex Protocols and Enhancing Consent
Long, technical consent forms and complicated trial procedures can overwhelm participants, leading to low enrollment and high dropout rates.
Solutions:
Streamlining trial protocols to minimize unnecessary procedures and visits is essential. Employing innovative consent processes, such as electronic consent with multimedia explanations and comprehension checks, can enhance understanding and engagement. Designing patient-centric study schedules that accommodate participants’ daily lives and offering clear and ongoing communication throughout the trial can improve retention rates. Utilizing patient feedback to refine protocols and procedures can ensure they are acceptable and manageable. Ensuring compliance with global standards like ICH Good Clinical Practice (GCP) is also critical for ethical and effective trial conduct.
Enhancing Physician Engagement and Reducing Referral Bias
Physicians often remain unaware of ongoing trials or hesitate to refer patients due to time constraints or unconscious bias.
Solutions:
Abiogenesis Clinpharm actively educates healthcare providers about current studies and integrates trial information into Electronic Health Records (EHRs). By involving site teams early during protocol development, we ensure that our recruitment strategies are practical and effective. We also provide incentives for referrals and deliver bias training to ensure ethical, equitable participation.
Streamlining Regulatory and Administrative Processes
Lengthy approval timelines and complex reporting requirements delay trial initiation and frustrate researchers and sponsors alike.
Solutions:
As one of the most reliable clinical research companies in Hyderabad, Abiogenesis Clinpharm promotes regulatory harmonization and leverages digital platforms for efficient data management and risk-based monitoring. Our proactive collaboration with regulators accelerates approvals and reduces administrative overhead.
Building Trust and Promoting Patient Engagement
Patients often worry about safety, data privacy, or feeling like mere test subjects. These concerns can lead to poor engagement and high attrition.
Solutions:
Transparency is the foundation of trust. We provide ongoing updates, return results to participants, and prioritize data privacy at every step. As a forward-thinking Clinical Research Organization, we involve patients in every phase—from trial design to results dissemination. Creating an environment where participants feel respected and heard has significantly improved retention across our studies.

Why Choose Abiogenesis Clinpharm?
Abiogenesis Clinpharm has been a consistent performer among clinical research companies in India, having successfully completed numerous studies across therapeutic areas. Based in Hyderabad, we are a leading Clinical Research Organization in Hyderabad with a track record of delivering reliable and high-quality services. Whether you’re a sponsor, investigator, or patient advocate, our commitment to seamless, ethical, and inclusive research makes us your ideal partner.
Know More About Clinical Research Organization
Looking for a trusted Clinical Research Organization in Hyderabad?
Partner with Abiogenesis Clinpharm—one of the top Clinical Research Organizations in India with over a decade of experience. For collaborations and inquiries, contact us at [email protected].

